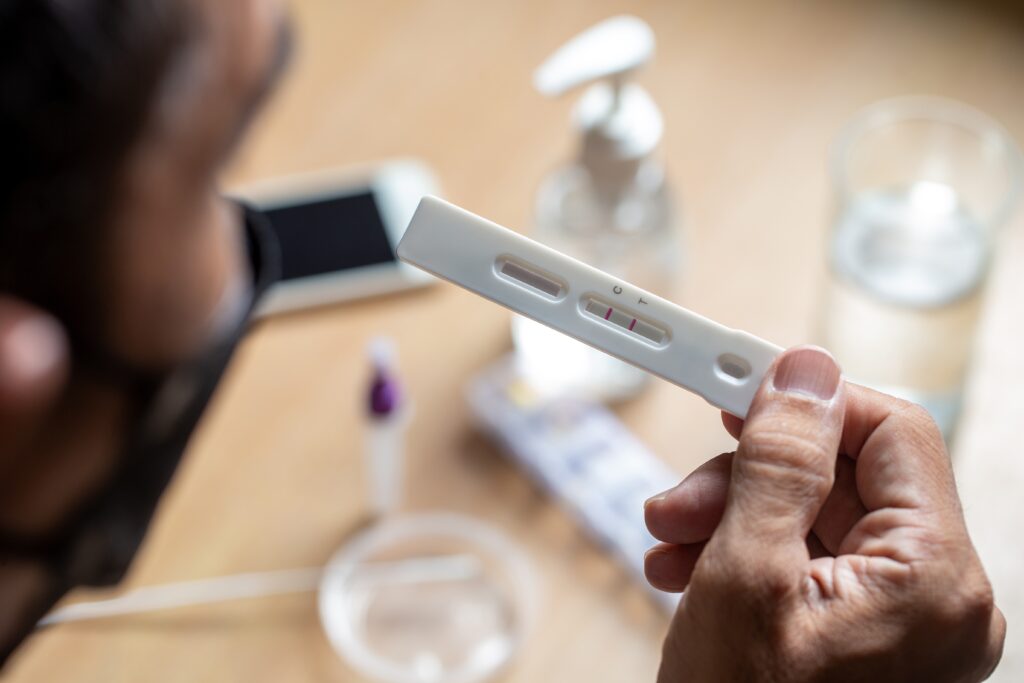
What Counts as a “Home Lab Test”?
Home-use tests let you collect a sample, or sometimes also run the test, outside of a clinic. Many of us have already used a home lab test by testing for COVID-19 using the at-home antigen testing kit. Other common examples include pregnancy tests, HIV self-tests, and stool-based colorectal cancer screening.
Some home lab tests are fully over-the-counter (OTC), while others involve mailing your sample to a CLIA-certified lab. The U.S. FDA regulates many of these tests and publishes consumer guidance on choosing and using them correctly.
Benefits of Home Lab Tests
- Speed & convenience: Immediate answers for time-sensitive questions (e.g., COVID-19) to help guide isolation and treatment.
- Privacy: HIV self-testing at home can reduce stigma barriers to screening and speed linkage to care.
- Prevention at scale: Stool-based colorectal cancer screening (FIT, stool DNA) allows broad outreach beyond colonoscopy, aligning with USPSTF recommendations to start screening at age 45.
The Fine Print: Limits and Risks
No test is perfect – false negatives, and false positives occur. For COVID-19 antigen tests, FDA advises repeat testing after a negative result to lower false-negative risk. Many combo or molecular home tests remain EUA-authorized (which means they are not fully approved) and must be used exactly as labeled. An important step in doing at-home tests is confirming any unexpected results or positives with a clinician.
As mail-in commercial panels vary in analytical rigor and clinical relevance, there is now a 2024–2025 policy shift that means more laboratory-developed tests (LDTs) will come under FDA oversight in coming years, aiming to improve safety and reliability.
While taking a home test has many benefits to provide information about your own health, clinically meaningful use and interpretation should involve your healthcare provider.
Where Home Tests Fit Best
- Infectious symptoms now (test-and-treat): COVID-19/flu home tests support quick decisions and telehealth prescriptions when indicated.
- Routine screening when guidelines allow: Annual FIT or stool DNA every 1–3 years is recommended for average-risk adults who decline or defer colonoscopy. Follow positive home results with colonoscopy—no exceptions.
- Private, targeted screening: FDA-approved HIV self-tests can be a first step; positive results need confirmatory testing and care linkage.
Smart Use: How to Avoid Pitfalls
- Buy FDA-authorized/approved tests from reputable sellers and avoid resold or foreign-market strips.
- Read the full instructions before swabbing – time and temperature matter! Keep records and don’t alter medications or make significant changes based solely on a home result.
- For antigen tests, retest 48 hours after a negative if suspicion remains. Always loop in a clinician for positive, persistent, or confusing results.
Bottom Line
There are highly recommended home tests for guideline-supported screening (e.g., colorectal cancer) and time-sensitive infectious decisions (COVID-19/flu), provided you choose FDA-authorized options, follow directions exactly, and confirm unexpected results with your healthcare provider. As FDA oversight tightens on LDTs, quality should improve further – good news for DIY health!
At Home Lab Tests Available:
- FDA list of OTC COVID-19 antigen tests (check brands and expiration updates).
- Lucira by Pfizer COVID-19 & Flu Home Test (molecular; EUA).
- OraQuick HIV Self-Test (OTC oral-fluid antibody test; FDA-approved).
- Colorectal cancer screening, at home
- FIT (annual) — see CDC screening overview to select a kit through your clinician/insurer.
- Cologuard® Plus stool DNA (every 3 years for average-risk adults 45+) — FDA PMA with patient labeling. FDA Access Data+1
References:
Centers for Disease Control and Prevention. (2025). Screening for colorectal cancer. https://www.cdc.gov/colorectal-cancer/screening/ CDC.
Food and Drug Administration. (2017). How you can get the best results with home use tests.
https://www.fda.gov/medical-devices/home-use-tests/how-you-can-get-best-results-home-use-tests.
Food and Drug Administration. (2023, Feb 24). FDA authorizes first over-the-counter at-home test to detect both influenza and COVID-19 viruses (Lucira). https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-over-counter-home-test-detect-both-influenza-and-covid-19-viruses.
Food and Drug Administration. (2024, Oct 3). Summary of safety and effectiveness data: Cologuard Plus™ (P230043). https://www.accessdata.fda.gov/cdrh_docs/pdf23/P230043B.pdf.
Food and Drug Administration. (2024, Dec 16). OraQuick HIV Self-Test (BP120001). https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/oraquick-hiv-self-test.
Food and Drug Administration. (2025). Home use tests (consumer hub). https://www.fda.gov/medical-devices/in-vitro-diagnostics/home-use-tests.
Food and Drug Administration. (2024, May 6). Medical devices; Laboratory developed tests—Final rule (Federal Register). https://www.federalregister.gov/documents/2024/05/06/2024-08935/medical-devices-laboratory-developed-tests.
Written By: Francis Ilag